Explain the Difference Between Exothermic and Endothermic
An easy way to remember the difference between these two reaction types is by their prefixes. 5 rows The major difference between endothermic and exothermic reactions as their names suggest is.
Difference Between Endothermic And Exothermic Reactions Definition Properties Examples
What is exothermic and endothermic reaction explain with example.
. Molar massMM of trinitroglycerin 227 gmol Mass of trinitroglycerin 575 g Moles of question_answer Q. When heat flows out of matter what happens. The difference between esothermic and endothermic.
This is possible due to cohesive forces. Therefore an endothermic reaction can be considered to any chemical reaction that is capable of absorbing energy. In endothermic processes reactants possess lower potential energy than the product.
3 marks 2 Explain how a catalyst changes the rate of a reaction. In a system energy can do work. Positive ΔH absorbs heat feels cold.
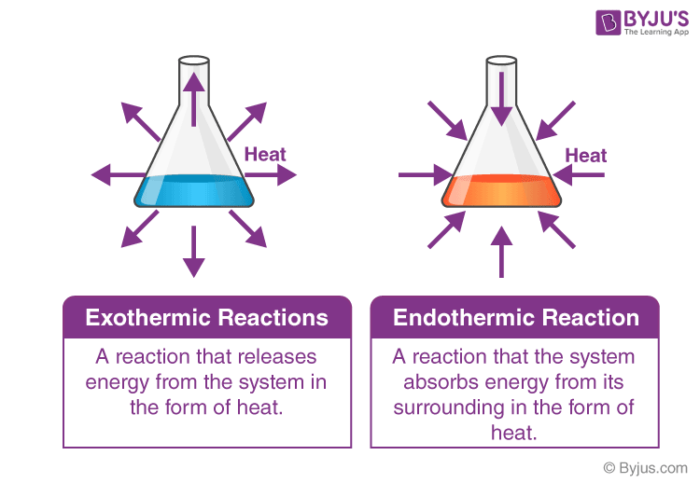
It can change into other forms such as heat sound light etc. Energy is the capacity to do work. The endothermic reactions are when the system takes up the energy in the form of light or heat.
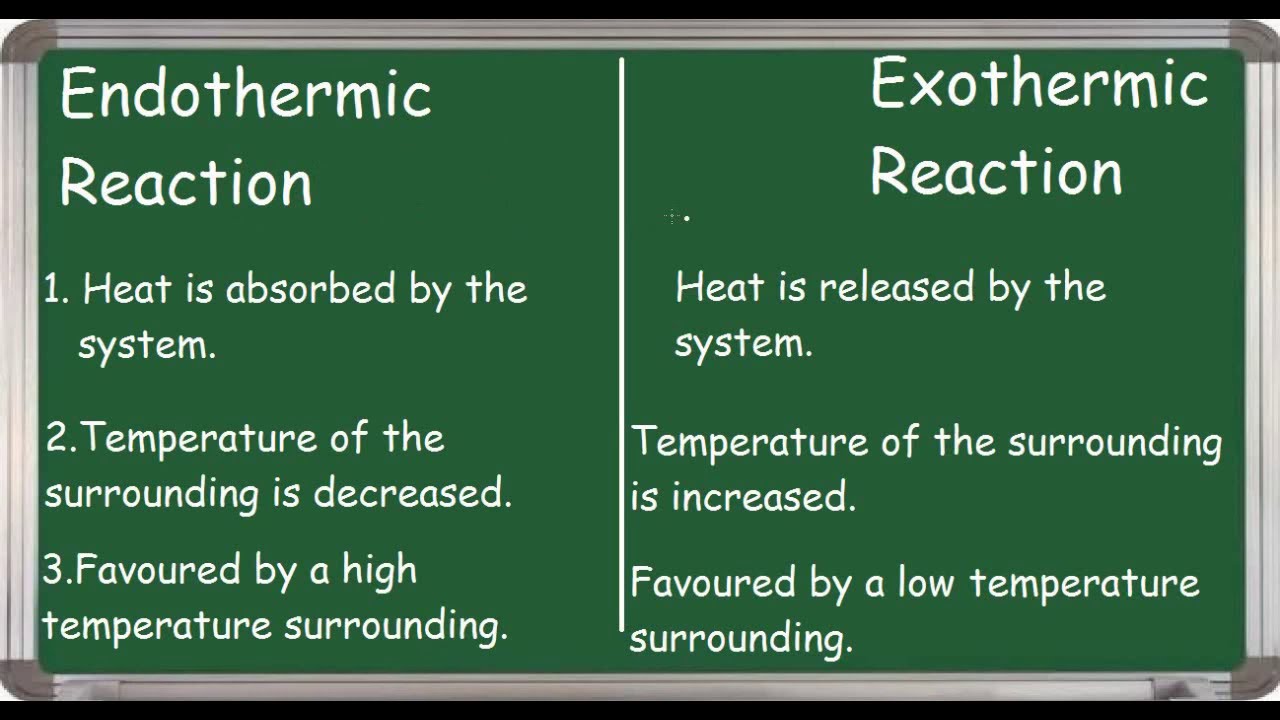
An endothermic reaction feels cold to the touch. What symbol is used to denote heat. The key difference between endothermic and exothermic reactions is that endothermic reactions absorb energy from the surrounding environment whereas exothermic reactions release energy to the surrounding environment.
A good example of an endothermic reaction is photosynthesis. What equation is used to represent enthalpy. Endo- means to draw in and exo- means to give off.
When a reaction proceeds it either releases energy to or absorbs energy from its surroundings. 5 rows In simple terms the endothermic reactions absorb energy from the surrounding that is in the form. An exothermic reaction releases energy and feels warm while an endothermic reaction absorbs energy and feels cool.
The main difference is that exothermal reactions release energy in the surroundings while an endothermic reaction absorbs energy from the surroundings. Photosynthesis is a popular example of an endothermic chemical reaction. Show your understanding using an energy potential diagram.
Once started exothermic reactions tend to keep going as each reaction releases more energy to fuel neighboring molecules. During this process. An exothermic reaction has a negative ΔH and gives off heat to the surroundings.
Negative ΔH gives off heat to surroundings feels warm Endothermic. How does enthalpy change in endothermic and exothermic reactions. Endothermic is the exact opposite.
In thermodynamics these two types of reactions are classified as exothermic or endothermic respectively. 15 Surface tension is property that allows liquids to repel or resist external forces. An endothermic process absorbs heat and cools the surroundings Based on the above definition lets pick a few examples from our daily lives and categorize them as endothermic or exothermic.
Thus in order to react they absorb the energy from the environment. An exothermic reaction feels warm to the touch. Endothermic is a reaction that need to be supplied with energy.
Endothermic reactions If forming new bonds in the products releases less energy than it took to break the original bonds the reaction is endothermic Endothermic reactions An important endothermic reaction is photosynthesis. 1 Explain the difference between an EXOTHERMIC reaction and an ENDOTHERMIC reaction. An exothermic process releases heat causing the temperature of the immediate surroundings to rise.
In the first heat is absorbed. Here are a number of highest rated What Is Endothermic Reaction pictures on internet. Enthalpy energy reactant products.
Upload your study docs or become a. Explain the difference between an exothermic and an endothermic reaction. An endothermic reaction has a positive ΔH and absorbs heat from the surroundings.
Endothermic and exothermic reactions are chemical reactions that absorb and release heat respectively. Remember to explain the difference using scientific terms such as. An exothermic reaction creates heat whereas a endothermic reaction absorbs heat.
Exothermic take place when two substances are mixed together and generated heat. Difference Between Endothermic and Exothermic Reactions Definition. In the second heat is given out.
We agree to this kind of What Is Endothermic Reaction graphic could possibly be the most trending subject in imitation of we share it in google lead or facebook. Conclusion this esothermic and endothermic reactions can be classified on the basis of the transfer of energy between the system and the surrounding environment. Want to read all 3 pages.
The temperature decrease with the progression of endothermic reactions. In contrast exothermic systems give up heat or light energy as the reaction proceeds. Endothermic reactions are chemical reactions that absorb heat energy from the.
14 Exothermic reactions give off energy and usually have stronger products than reactants. An exothermic reaction has an overall negative enthalpy change and releases heat to its question_answer Q. We identified it from honorable source.
Enthalpy Chemists use enthalpy instead of heat to describe the energy transfer. Difference between Endothermic and Exothermic Reaction 1. The difference between Endothermic and Exothermic energy is that Exothermic energy is the reaction that RELEASES energy and Endothermic is the reaction in which energy is ABSORBED.
Its submitted by executive in the best field. When heat flows into matter what happens. Consequently in the endothermic reactions the enthalpy of the products is greater positive than that of the reactants ie the exothermic reactions will present positive enthalpy differences due to the energy they absorb.
Endothermic Vs Exothermic Reaction Differences Youtube
Difference Between Endothermic And Exothermic Reactions Chemistry

7 Difference Between Exothermic And Endothermic Reaction With Examples Viva Differences
Belum ada Komentar untuk "Explain the Difference Between Exothermic and Endothermic"
Posting Komentar